
Effect of (a) NaI and (b) MnO2 concentration on H2O2 decomposition.... | Download Scientific Diagram

25 ml of 0.50M H2O2 solution is added to 50ml of 0.20M | Equivalence weight | Equivalance master - YouTube
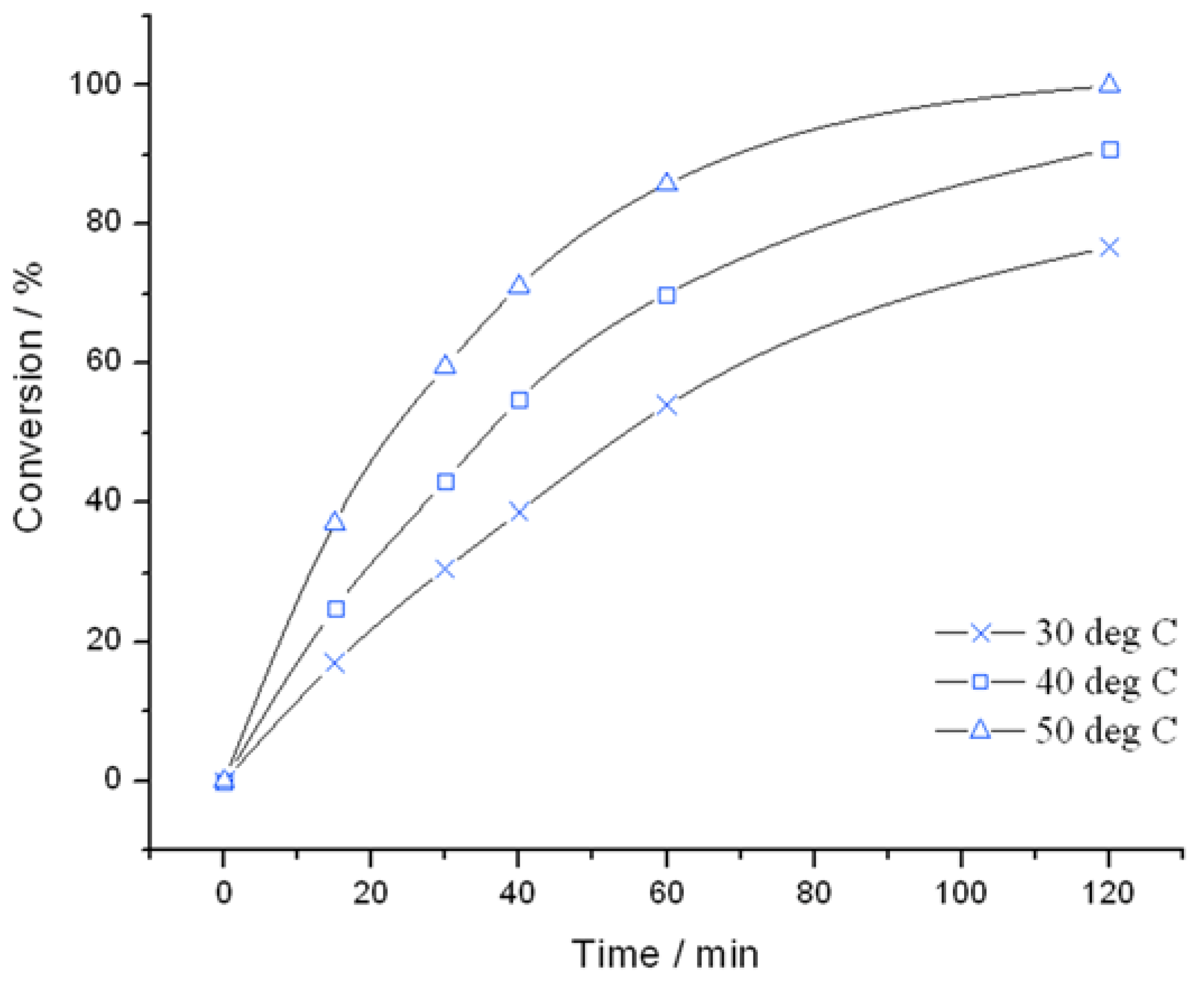
Catalysts | Free Full-Text | Switching off H2O2 Decomposition during TS-1 Catalysed Epoxidation via Post-Synthetic Active Site Modification

The volume strength of 8.9 M H2O2 solution calculated at 273 K and 1 atm is ………. (R = 0.0821 L atm K - YouTube

In situ H2O2 Generation and Corresponding Pollutant Removal Applications: A Review - Ji - 2023 - Chemistry – A European Journal - Wiley Online Library
![Generation of HO• Radical from Hydrogen Peroxide Catalyzed by Aqua Complexes of the Group III Metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A Theoretical Study | ACS Catalysis Generation of HO• Radical from Hydrogen Peroxide Catalyzed by Aqua Complexes of the Group III Metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A Theoretical Study | ACS Catalysis](https://pubs.acs.org/cms/10.1021/cs400155q/asset/images/medium/cs-2013-00155q_0021.gif)
Generation of HO• Radical from Hydrogen Peroxide Catalyzed by Aqua Complexes of the Group III Metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A Theoretical Study | ACS Catalysis
![Generation of HO• Radical from Hydrogen Peroxide Catalyzed by Aqua Complexes of the Group III Metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A Theoretical Study | ACS Catalysis Generation of HO• Radical from Hydrogen Peroxide Catalyzed by Aqua Complexes of the Group III Metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A Theoretical Study | ACS Catalysis](https://pubs.acs.org/cms/10.1021/cs400155q/asset/images/large/cs-2013-00155q_0005.jpeg)
Generation of HO• Radical from Hydrogen Peroxide Catalyzed by Aqua Complexes of the Group III Metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A Theoretical Study | ACS Catalysis

SOLVED: Calculate the volume (mL) of 30.0% (w/w) H2O2 needed to prepare 50.0 mL of 3.6 M H2O2. The density of 30% (w/w) H2O2 is 1.11 g/mL at 25°C. Note: 30% (w/w)


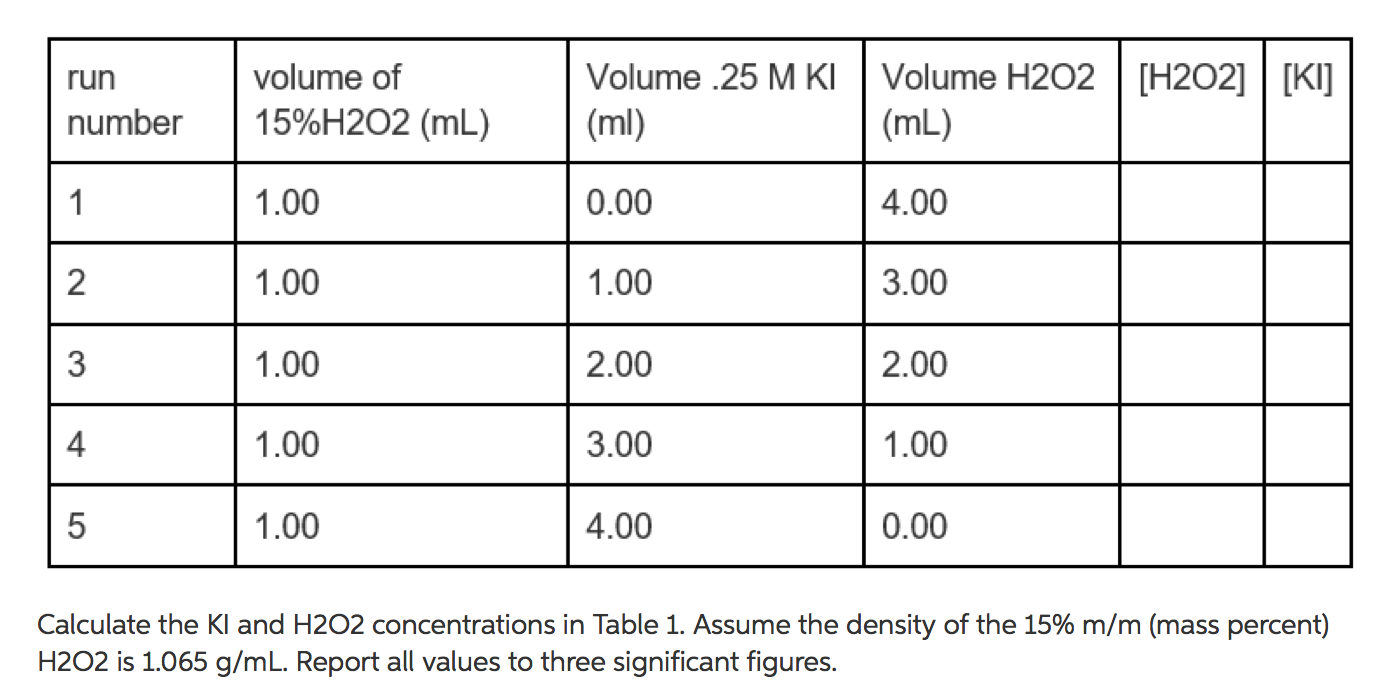


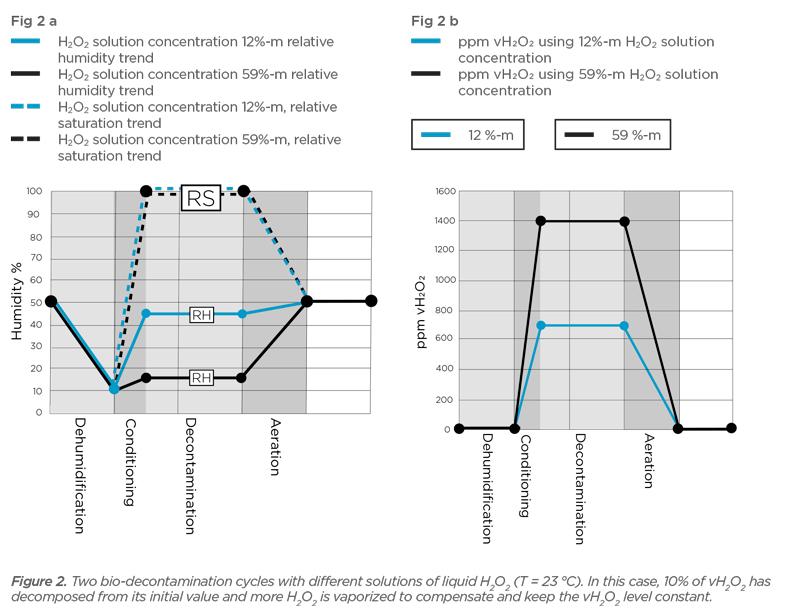

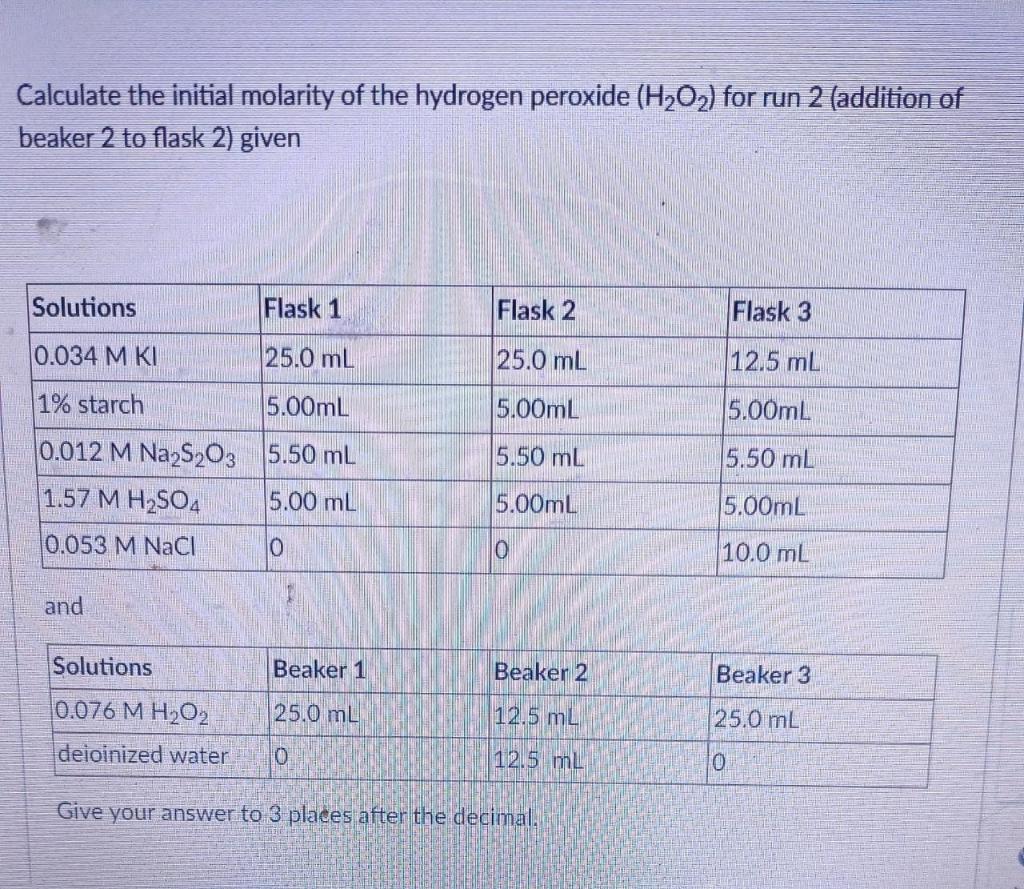


![The volume strength of 1 M H2O2 is:[Molar mass of H2O2 = 34 g mol^-1 ] The volume strength of 1 M H2O2 is:[Molar mass of H2O2 = 34 g mol^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/2014383/fac00b3a-19e7-4014-9ac0-61169e75d5e8.jpg)